Early this morning the US/German biotech Agennix announced a significant restructuring whereby it would attempt to spare capital by dismissing 55% of its workforce, or 37 people. The restructuring isn't a surprise; two weeks ago Agennix said its lead program in non-small cell lung cancer failed a Phase III trial in the third-line setting. The company's market value has deteriorated accordingly; it now trades at or below the Eur22.7 million in cash it had as of the end of June. It'll certainly need a financial infusion if it plans to continue any operations beyond the first quarter of 2013. What are the odds investors buck up?
Though clearly a disappointment for patients, Agennix management, and its unusually concentrated investor base (the company is publicly traded but controlled by shareholder Dietmar Hopp, whose funds hold about 65% of Agennix's stock), the biotech's plight provides a timely illustration of some fairly common biotech problems. And it allows us to point toward a theoretical solution.
Agennix, the product of a merger of two biotechs in 2009, is a somewhat diversified company, but investors obviously based its entire value on that Phase III program. Besides developing talactoferrin alfa -- the above-mentioned immunotherapy that failed in early August -- in two NSCLC settings, other cancer indications, severe sepsis, and (as a topical formula) in diabetic foot ulcers, Agennix also has rights to a handful of unrelated oncology programs (remember satraplatin?). The biotech is far from alone in this kind of pipeline-in-a-product diversification, even if it is unusually stretched -- from lung to foot -- to the physiological maximum. It's also about average when it comes to the way investors viewed its later-stage programs (as in, if it ain't first, it don't matter). In fact, more or less, this is the default biotech valuation model.
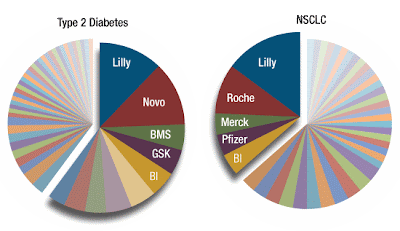
Check out the twin pie charts at the top of this post, created with data provided by TechAtlas. At least 48 different biopharmaceutical companies are pursuing clinical-stage treatments for Type II diabetes. In NSCLC, 41 different companies have drugs that are in Phase II or beyond. Only a handful of competitors – typically the largest companies – are pursuing multiple agents against the same disease. Most NSCLC developers are like Agennix -- small, diversified, and reliant upon a single asset because it's first in the firing line. It's the larger companies, those with multiple drug candidates in development for the same indication, that therefore are able not only to identify the best single agent of the group but also to experiment with combinations pre-commercialization.
But imagine a parallel universe version of a company like Agennix, one that was diversified around a single indication instead, say NSCLC, before it reached pivotal studies. A company that instead of chasing three or four disparate indications, was wholly focused on one well-defined problem. And instead of relying heavily on a single asset and a series of clinical trials that pits that asset against placebo or standard of care, it had a handful of candidates against that single indication in the same stage of development, and tested them against one another in a proof-of-concept trial.
Wouldn't investors have more faith that the winner of that trial not just in terms of its ability to succeed in Phase III, but the likelihood that it passes muster with regulators and payors as well? Wouldn't an ecosystem populated by these solution-focused companies reduce duplicative clinical infrastructure, find it easier to recruit patients to participate in clinical trials, and attract likeminded and driven backers?
Such are the arguments that RA Capital's Peter Kolchinsky puts forth in the September issue of IN VIVO (subscribers should check out the full feature here; we'll also be discussing this model on a panel at our upcoming Pharmaceutical Strategic Alliances meeting -- Sept 17-19 in NYC).
Pulling together the foundations of a so-called Solution Development company is a tall though by no means impossible order. Beyond the central arguments about how to build biotechs and design meaningful trials, Kolchinsky suggests a role for disease foundations in identifying promising assets and even incentivizing developers to pool their candidates, as well as the opportunity for large pharmaceutical players to strike pre-negotiated deals to license the winners of these contests.
For the whole feature, get over to IN VIVO. Meanwhile we've got the solution to your late-summer Friday blues, it's time for the latest installment of ...
Allergan/Molecular Partners: Allergan must like what it sees in Molecular Partners' obscurely named DARPins, returning Aug. 21 to license another potential therapy for wet age-related macular degeneration (AMD), MP0260, and entering into a discovery collaboration to find more DARPin compounds with activity against serious eye conditions. The U.S. ophthalmics-to-dermatology specialty company already has licensed the Swiss biotech's lead compound, MP0112 (AGN-150998), which is being compared with Genentech/Novartis' Lucentis (ranibizumab) in a Phase IIb clinical study. DARPins are based on repeated peptide sequences that mediate natural protein-protein interactions and have some interesting characteristics, including a possibly longer duration of action in the eye than current AMD therapies like Lucentis and Regeneron/Bayer's Eylea (aflibercept), and maybe improved efficacy. In return for access to the cutting-edge research, Allergan is making an upfront payment of $62.5 million for the two new agreements, which include licensing options on three compounds. In total, Molecular Partners could receive up to an eye-watering $1.4 billion in development and regulatory milestones and tiered royalties on future product sales. The company signed up Janssen Biotech in December 2011 to evaluate DARPins for immunological applications, and also is developing its own pipeline of proprietary products.—John Davis
Riemser Arzneimittel AG/AXA Private Equity: The Greifswald, Germany-based specialty pharmaceutical company Riemser Arzneimittel has been sold by the founding Braun family and various minority shareholders, including TVM Capital, to the European diversified private equity firm, AXA Private Equity, for an undisclosed amount, the companies announced Aug. 21. Over the past 30 years, Riemser has made a remarkable journey, from a veterinary vaccines company based in East Germany to an international marketer of niche pharmaceuticals in the oncology, anti-infectives and dermatological fields. This has been achieved by organic growth and by the acquisition of smaller German companies and portfolios of unwanted products from other companies, supported by investments from TVM Capital and GE Capital. Riemser divested its veterinary business in 2010. AXA Private Equity intends to use its global network to support Riemser's internationalization strategy and its continued focus on niche therapeutics. A new round of company consolidation also might be in the cards in this less-risky corner of the pharmaceutical industry.—J.D.
Silence Therapeutics/MiReven: Silence Therapeutics has been tapped again for its RNA-delivery techniques that allow RNA-based drugs to reach tissues deep within the body. Australian-based microRNA company MiReven has signed a deal with Silence to use its delivery system with its cancer drug miR-7. Silence will formulate a miR-7 mimetic molecule with its proprietary lipid-delivery systems in order to evaluate miR-7 in various cancer models. It will also undertake in vitro and in vivo studies of the formulated miR-7. Silence is being paid an undisclosed fee for the collaboration. In July, Silence raised $8.8 million from new and existing investors to help extend its cash runway to 2014. The company uses its revenue from delivery partnerships, as well as funds raised to forward its own internal pipeline, which includes RNA-interference compounds. Silence Chief Scientific Officer Klaus Giese noted that this is the company’s fourth microRNA delivery technology partnership. “Whilst we remain internally focused on the delivery of our siRNA therapies, we continue to broaden the potential value of our proprietary delivery systems by collaborating with partners,” he added.—Lisa LaMotta
Pfizer/Mylan: In one corner, Pfizer wanted to expand its Established Products footprint in Japan, the world’s second-largest pharma market but only the sixth-largest generics market with room to grow. In the other, Mylan has more than 380 products in its portfolio in Japan, but has been stalled in gaining significant market share due to lacking distribution and marketing capabilities. The two firms say it was a perfect fit for an “exclusive, long-term strategic collaboration” for generics in Japan. Pfizer will slap its label on Mylan’s existing portfolio and an additional 125 compounds in Mylan’s pipeline and handle the commercial side, while Mylan is tasked with development and manufacturing of compounds in the agreement. A key to success in gaining market share in Japan’s generic market is securing preference from nationwide wholesalers. Pfizer’s status as an innovative company likely will go a long way in bumping Mylan-sourced products near the top of wholesalers’ recommendation lists for hospital sales. The companies will split revenue, but otherwise the deal does not include any capital alliance. In terms of sales, Pfizer Established Products Business Unit President Albert Bourla said the firms have “very high ambitions” to be a market leader in terms of sales by 2015.—Daniel Poppy
Bristol-Myers Squibb/Synergy: Gastrointestinal disorder-focused Synergy Pharmaceuticals signed an asset purchase agreement Aug. 23 with Bristol-Myers Squibb to acquire all assets related to FV-100, an orally available nucleoside analogue in mid-stage testing for shingles. Financial terms were not disclosed. Bristol previously had completed a Phase IIa trial of the candidate in which it was found to be well tolerated dosed at 200 mg and 400 mg in 230 patients. The trial also demonstrated clinically meaningful reductions in time to resolution of clinically significant pain and in incidence of post-herpetic neuralgia, Synergy stated in a release. "We believe that with our expanding clinical experience in utilizing patient-reported outcome tools from our GI program, a feature that will be necessary for supporting pain-related indications for FV-100, we are in a unique position to further develop FV-100 for patients not adequately treated with present-day therapy,” said Synergy CEO Gary Jacob. In July, Synergy merged with cancer-focused Callisto Pharmaceuticals, its largest shareholder, in a tax-free stock swap.—Joseph Haas
Friday, August 24, 2012
Deals of the Week Points to a Solution to Your Phase III Woes
By
Chris Morrison
at
1:34 PM
![]()
Labels: alliances, business models, deals of the week, research and development strategies
Subscribe to:
Post Comments (Atom)


No comments:
Post a Comment